Delving into the Heart of Matter: Unveiling the Structure of the Atom
Related Articles: Delving into the Heart of Matter: Unveiling the Structure of the Atom
Introduction
With great pleasure, we will explore the intriguing topic related to Delving into the Heart of Matter: Unveiling the Structure of the Atom. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Delving into the Heart of Matter: Unveiling the Structure of the Atom

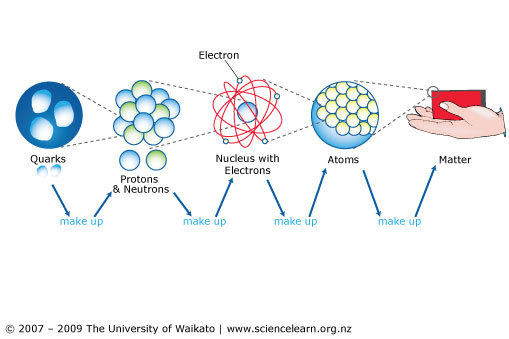
The atom, the fundamental building block of all matter, has captivated the minds of scientists for centuries. Its intricate structure, once thought to be indivisible, now reveals a fascinating world of subatomic particles, governed by the principles of quantum mechanics. Understanding the makeup of the atom is crucial for comprehending the nature of the universe, from the formation of stars to the behavior of everyday materials.
A Journey into the Atom’s Core
At the heart of every atom lies the nucleus, a dense and positively charged region containing two types of particles: protons and neutrons.
-
Protons, carrying a single positive charge, determine the element to which an atom belongs. The number of protons, known as the atomic number, defines the element’s identity. For instance, all carbon atoms have six protons, while all oxygen atoms have eight.
-
Neutrons, carrying no charge, contribute to the atom’s mass. The total number of protons and neutrons within the nucleus is known as the mass number. While the number of protons is fixed for a given element, the number of neutrons can vary, leading to the existence of isotopes, atoms of the same element with different masses.
The Electron Cloud: A Realm of Uncertainty
Surrounding the nucleus is a vast, negatively charged region known as the electron cloud. This cloud is not a solid structure but rather a probabilistic region where electrons, each carrying a single negative charge, reside. The electrons are constantly in motion, their positions and momenta governed by the principles of quantum mechanics.
Electrons occupy specific energy levels, or shells, around the nucleus. Each shell can accommodate a limited number of electrons, with higher shells corresponding to higher energy levels. The arrangement of electrons in these shells determines an atom’s chemical properties and its ability to form bonds with other atoms.
The Quantum Mechanical Model: A Revolution in Atomic Understanding
The modern understanding of the atom relies heavily on the principles of quantum mechanics, a revolutionary theory that describes the behavior of matter at the atomic and subatomic levels. This model, developed in the early 20th century, challenged the classical view of the atom as a miniature solar system with electrons orbiting the nucleus like planets.
-
Wave-Particle Duality: Quantum mechanics posits that electrons exhibit both wave-like and particle-like behavior. This means that electrons can act as both particles with definite positions and momenta, and as waves that can interfere and diffract.
-
Quantized Energy Levels: The electrons in an atom can only exist in discrete energy levels, meaning they cannot occupy any energy value between these levels. This quantization of energy levels explains the characteristic spectral lines observed when atoms absorb or emit light.
-
Probability Distribution: The electrons in an atom are not confined to specific orbits but rather occupy a probability distribution, meaning their exact position and momentum cannot be simultaneously known. This concept is embodied in the Heisenberg uncertainty principle.
The Atom’s Importance: From Matter to Life
The structure of the atom is not merely an abstract concept; it holds profound implications for understanding the world around us.
-
Chemical Reactions: The arrangement of electrons in an atom’s outermost shell dictates its chemical reactivity. Atoms tend to gain, lose, or share electrons to achieve a stable configuration, leading to the formation of chemical bonds and the creation of molecules. This process underpins all chemical reactions, from the formation of water to the complex processes of life.
-
Materials Science: The properties of materials, such as conductivity, strength, and reactivity, are directly linked to the atomic structure and the interactions between atoms. By manipulating the arrangement and bonding of atoms, scientists can create materials with specific properties for various applications, from semiconductors to advanced alloys.
-
Nuclear Physics: Understanding the nucleus is essential for exploring the field of nuclear physics, which delves into the forces that bind protons and neutrons together, the processes of nuclear fusion and fission, and the development of nuclear energy and medicine.
FAQs: Unveiling the Mysteries of the Atom
Q: What is the smallest particle of an element?
A: The smallest particle of an element that retains the chemical properties of that element is an atom. However, atoms themselves are composed of smaller subatomic particles, such as protons, neutrons, and electrons.
Q: How are atoms different from molecules?
A: Atoms are the fundamental building blocks of matter, while molecules are formed by the combination of two or more atoms held together by chemical bonds. For example, a water molecule (H₂O) is formed by the bonding of two hydrogen atoms and one oxygen atom.
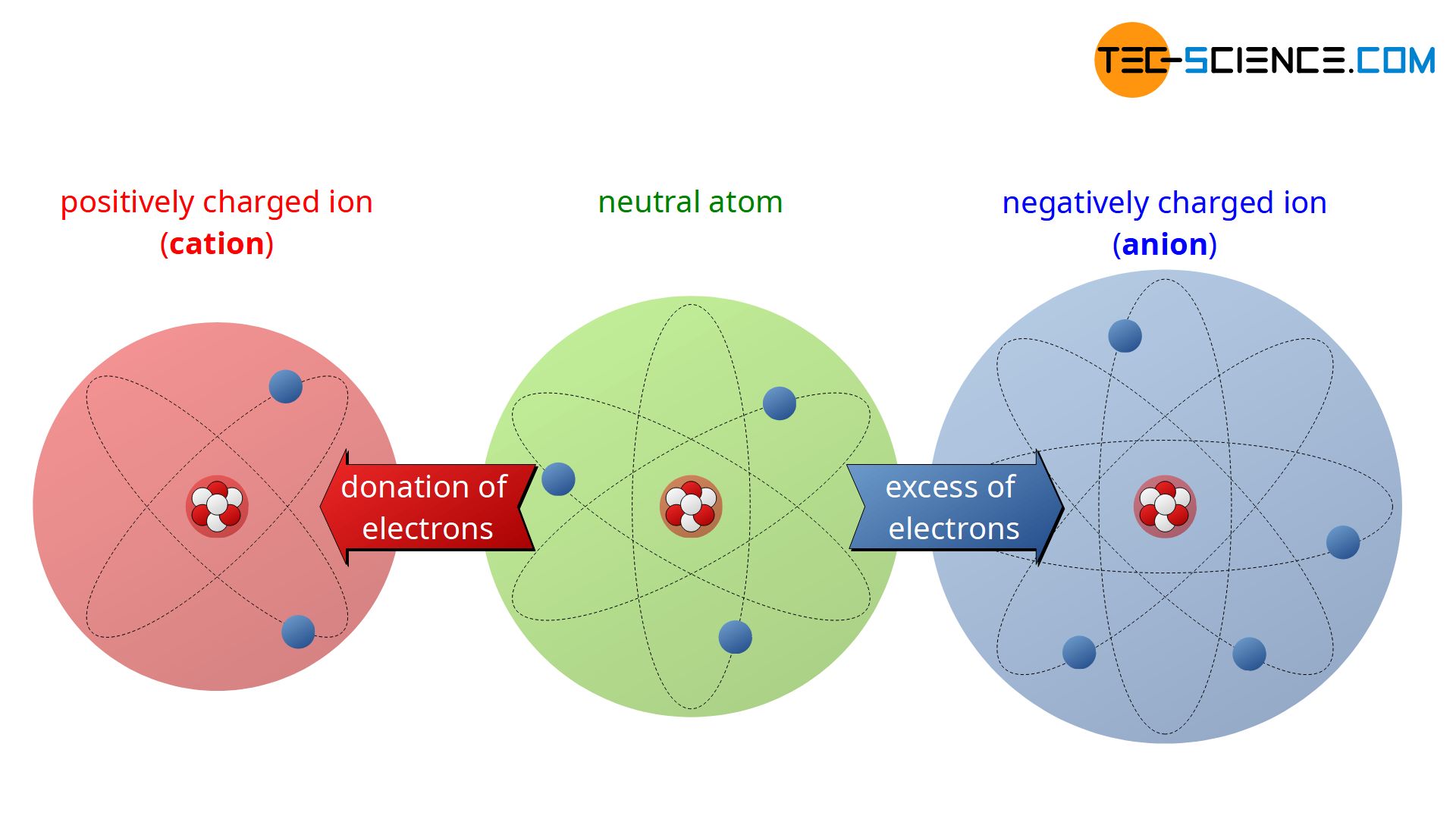
Q: What is the difference between an atom and an ion?
A: An atom is electrically neutral, containing an equal number of protons and electrons. An ion, on the other hand, is an atom that has gained or lost one or more electrons, giving it a net positive or negative charge.
Q: How is the atomic model related to the periodic table?
A: The periodic table is organized based on the atomic number, which represents the number of protons in an atom’s nucleus. The arrangement of elements in the periodic table reflects the recurring patterns in the electron configurations of atoms, explaining their chemical properties and reactivity.
Tips for Understanding the Atom
-
Visualize: Use diagrams and models to visualize the structure of the atom, including the nucleus, electron cloud, and energy levels.
-
Focus on the Basics: Start by understanding the key concepts of protons, neutrons, and electrons, their charges, and their roles in determining an atom’s identity and properties.
-
Embrace Quantum Mechanics: While the concept of quantum mechanics can be challenging, it is crucial for understanding the behavior of electrons and the nature of the atom.
-
Explore Applications: Connect the knowledge of atomic structure to real-world applications in fields like chemistry, materials science, and nuclear physics.
Conclusion: A Journey of Discovery Continues
The atom, once thought to be the smallest indivisible particle, has revealed a complex and fascinating world of subatomic particles and quantum phenomena. Understanding its structure and behavior is fundamental to unraveling the mysteries of the universe and harnessing the power of matter for the benefit of humanity. As scientific exploration continues, we can expect further discoveries and deeper insights into the intricate world within the heart of every atom.





Closure
Thus, we hope this article has provided valuable insights into Delving into the Heart of Matter: Unveiling the Structure of the Atom. We hope you find this article informative and beneficial. See you in our next article!