Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry
Related Articles: Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry
Introduction
With great pleasure, we will explore the intriguing topic related to Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry
- 2 Introduction
- 3 Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry
- 3.1 Understanding the Structure and Properties of DMAP
- 3.2 DMAP: A Catalyst for Enhanced Reactivity
- 3.3 Applications of DMAP in Organic Synthesis
- 3.4 Benefits of Using DMAP in Organic Synthesis
- 3.5 FAQs Regarding DMAP
- 3.6 Tips for Using DMAP Effectively
- 3.7 Conclusion: The Significance of DMAP in Modern Organic Synthesis
- 4 Closure
Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry

4-Dimethylaminopyridine, commonly known as DMAP, is a versatile and powerful reagent in organic chemistry. Its unique properties make it a valuable tool for accelerating and improving the efficiency of various chemical reactions, particularly those involving acylations. This article delves into the intricacies of DMAP, exploring its structure, mechanism of action, applications, and significance in modern organic synthesis.
Understanding the Structure and Properties of DMAP
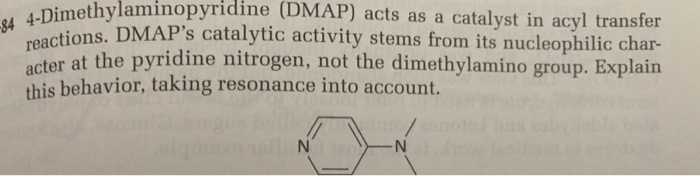
DMAP is a heterocyclic aromatic compound with a pyridine ring bearing a dimethylamino group at the 4-position. This structural feature plays a crucial role in its reactivity. The nitrogen atom in the dimethylamino group exhibits strong electron-donating properties, making the pyridine ring highly electron-rich. This electron density is delocalized across the ring, creating a resonance structure with a positive charge on the nitrogen atom.
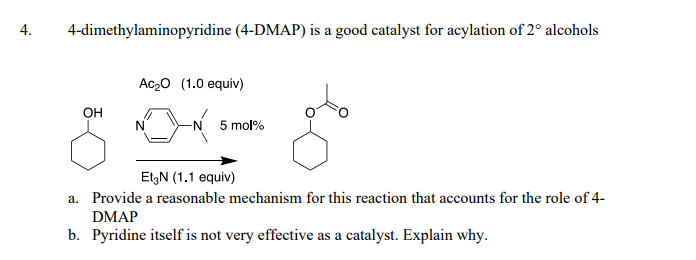
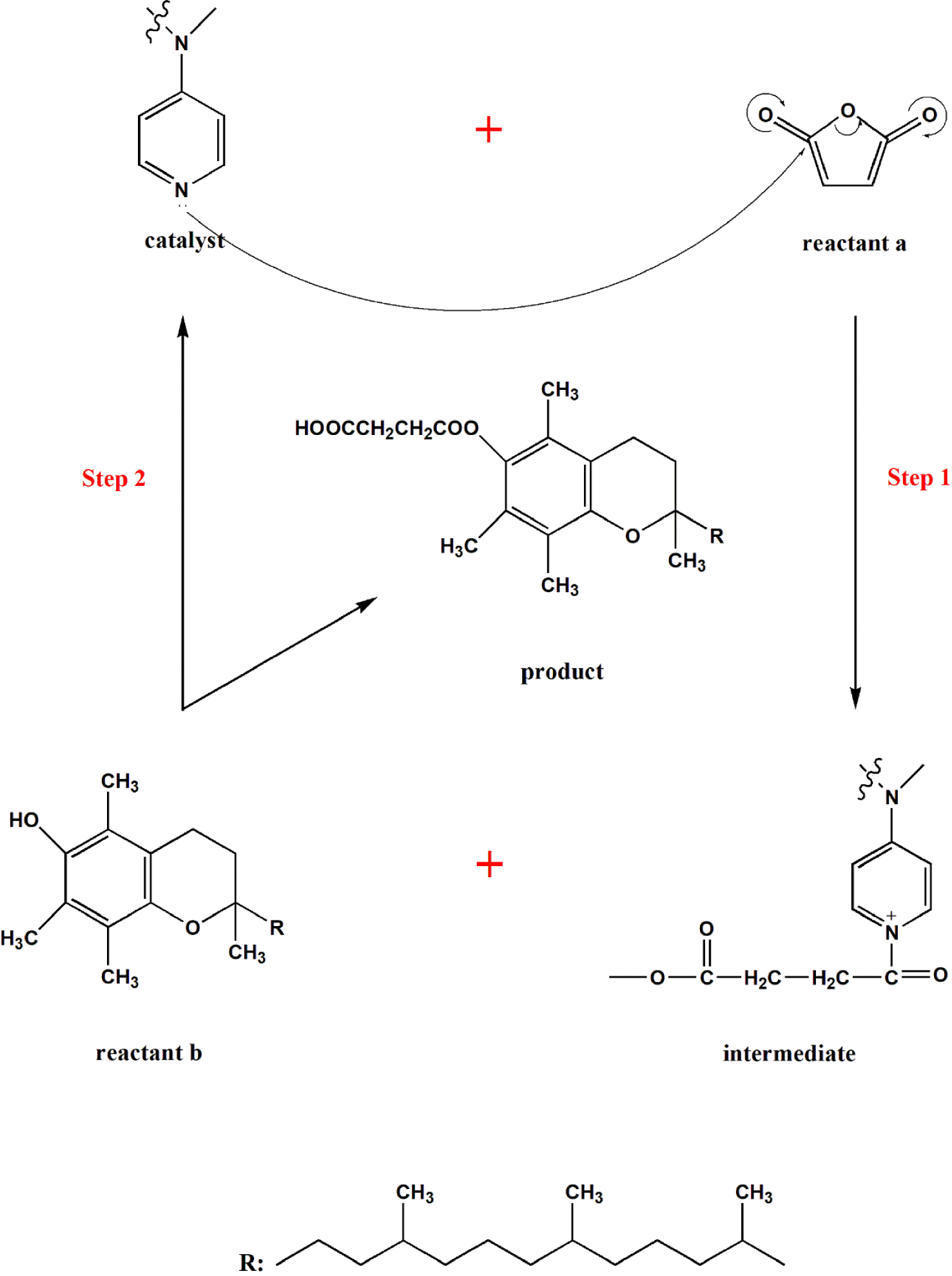
The electron-rich nature of the pyridine ring in DMAP is responsible for its catalytic activity. It acts as a nucleophile, readily interacting with electrophilic species like acyl halides or anhydrides. This interaction facilitates the formation of a highly reactive intermediate, which subsequently reacts with the nucleophile in the reaction mixture, leading to the desired product.
DMAP: A Catalyst for Enhanced Reactivity
DMAP’s ability to accelerate and enhance the efficiency of chemical reactions stems from its catalytic nature. It acts as a Lewis base catalyst, facilitating the formation of a more reactive intermediate and lowering the activation energy of the reaction. This effect can be attributed to the following factors:
- Nucleophilic Activation: DMAP’s electron-rich pyridine ring readily interacts with electrophilic species, forming a highly reactive intermediate. This intermediate is more susceptible to nucleophilic attack, leading to faster reaction rates.
- Stabilization of the Transition State: DMAP can stabilize the transition state of the reaction by forming a complex with the intermediate. This stabilization lowers the activation energy of the reaction, further accelerating the reaction rate.
- Increased Selectivity: DMAP can also enhance the selectivity of certain reactions, favoring the formation of specific products. This is particularly relevant in reactions involving multiple nucleophiles or electrophilic centers.
Applications of DMAP in Organic Synthesis
DMAP’s versatility and effectiveness have made it an indispensable tool in various organic synthesis applications. Some of the most prominent examples include:
- Acylation Reactions: DMAP is widely used to catalyze acylation reactions, including the formation of esters, amides, and anhydrides. Its ability to activate acyl halides and anhydrides makes it an efficient catalyst for these reactions.
- Esterification Reactions: DMAP is highly effective in promoting esterification reactions, particularly those involving sterically hindered alcohols or carboxylic acids. Its catalytic activity significantly improves the yield and reaction rate compared to traditional methods.
- Amide Formation: DMAP can be employed to catalyze the formation of amides from carboxylic acids and amines. Its ability to activate the carboxylic acid group facilitates the formation of the amide bond.
- Ring-Opening Reactions: DMAP can be used to catalyze ring-opening reactions of cyclic esters and lactones. Its nucleophilic properties enable it to interact with the electrophilic carbonyl group, initiating the ring-opening process.
- Other Reactions: DMAP has also been utilized as a catalyst in various other reactions, including alkylation, silylation, and ring-closing metathesis. Its versatility and effectiveness make it a valuable tool for a wide range of synthetic transformations.
Benefits of Using DMAP in Organic Synthesis
The use of DMAP in organic synthesis offers numerous benefits compared to traditional methods:
- Increased Reaction Rates: DMAP’s catalytic properties significantly accelerate the reaction rates, allowing for faster and more efficient synthesis.
- Improved Yields: The use of DMAP often leads to higher yields of desired products compared to uncatalyzed reactions.
- Enhanced Selectivity: DMAP can improve the selectivity of reactions, favoring the formation of specific products.
- Milder Reaction Conditions: DMAP can facilitate reactions under milder conditions, reducing the need for harsh temperatures or pressures.
- Reduced Waste Generation: The use of DMAP can minimize waste generation by improving reaction efficiency and reducing side reactions.
FAQs Regarding DMAP
Q: What are the safety considerations associated with DMAP?
A: DMAP is a moderately toxic compound and should be handled with caution. It is a skin and eye irritant and can cause respiratory irritation if inhaled. Proper handling procedures, including wearing gloves and a mask, are essential when working with DMAP.
Q: How is DMAP typically used in organic synthesis?
A: DMAP is typically used as a catalyst in organic reactions. It is often added in small amounts to the reaction mixture, typically in the range of 1-10 mol%.
Q: What are the common side reactions associated with DMAP?
A: DMAP can participate in side reactions, such as the formation of N-acyl-DMAP intermediates. These side reactions can be minimized by optimizing reaction conditions and using appropriate stoichiometry.
Q: What are the alternatives to DMAP in organic synthesis?
A: Several alternatives to DMAP exist, including other pyridine-based catalysts like 4-pyrrolidinopyridine (PPY) and 4-picoline. The choice of catalyst depends on the specific reaction and desired outcome.
Q: How can the effectiveness of DMAP be optimized in a reaction?
A: The effectiveness of DMAP can be optimized by adjusting reaction parameters like temperature, solvent, and the amount of catalyst used. It is crucial to consider the specific reaction and optimize conditions accordingly.
Tips for Using DMAP Effectively
- Use High-Quality DMAP: Ensure the DMAP used is of high purity to maximize its catalytic activity and minimize side reactions.
- Optimize Reaction Conditions: Adjust reaction parameters like temperature, solvent, and catalyst loading to optimize the reaction outcome.
- Consider the Reaction Stoichiometry: Use appropriate stoichiometry to minimize side reactions and maximize product yield.
- Monitor the Reaction Progress: Monitor the reaction progress using analytical techniques like TLC or NMR to ensure complete conversion and prevent over-reaction.
- Handle DMAP with Caution: Observe proper safety protocols when handling DMAP, including wearing gloves and a mask.
Conclusion: The Significance of DMAP in Modern Organic Synthesis
DMAP has emerged as a powerful and versatile reagent in modern organic synthesis. Its unique properties, including its ability to accelerate reaction rates, improve yields, and enhance selectivity, have made it an indispensable tool for a wide range of chemical transformations. Its use in acylation, esterification, amide formation, and other reactions has significantly improved the efficiency and effectiveness of these processes.
Despite its remarkable benefits, the use of DMAP requires careful consideration of its safety aspects and optimization of reaction conditions to maximize its effectiveness and minimize side reactions. Overall, DMAP remains a valuable and indispensable reagent in the arsenal of modern organic chemists, contributing significantly to the advancement of synthetic chemistry and the development of new and innovative compounds.





Closure
Thus, we hope this article has provided valuable insights into Demystifying the Power of 4-Dimethylaminopyridine (DMAP): A Catalyst for Enhanced Reactivity in Organic Chemistry. We hope you find this article informative and beneficial. See you in our next article!