Demystifying the Significance of Molecular Weight in Dimethylformamide (DMF)
Related Articles: Demystifying the Significance of Molecular Weight in Dimethylformamide (DMF)
Introduction
With great pleasure, we will explore the intriguing topic related to Demystifying the Significance of Molecular Weight in Dimethylformamide (DMF). Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Demystifying the Significance of Molecular Weight in Dimethylformamide (DMF)

Dimethylformamide (DMF) is a versatile organic solvent widely employed in various industrial and scientific applications. Its molecular weight, a crucial parameter influencing its properties and applications, plays a significant role in understanding its behavior and optimizing its use. This article delves into the intricacies of DMF’s molecular weight, exploring its importance, benefits, and implications across diverse fields.
Understanding Molecular Weight: A Fundamental Concept
Molecular weight, often referred to as molecular mass, represents the sum of the atomic masses of all atoms constituting a molecule. It is expressed in atomic mass units (amu) or grams per mole (g/mol). In the context of DMF, understanding its molecular weight is paramount for accurate calculations, precise chemical reactions, and efficient process control.
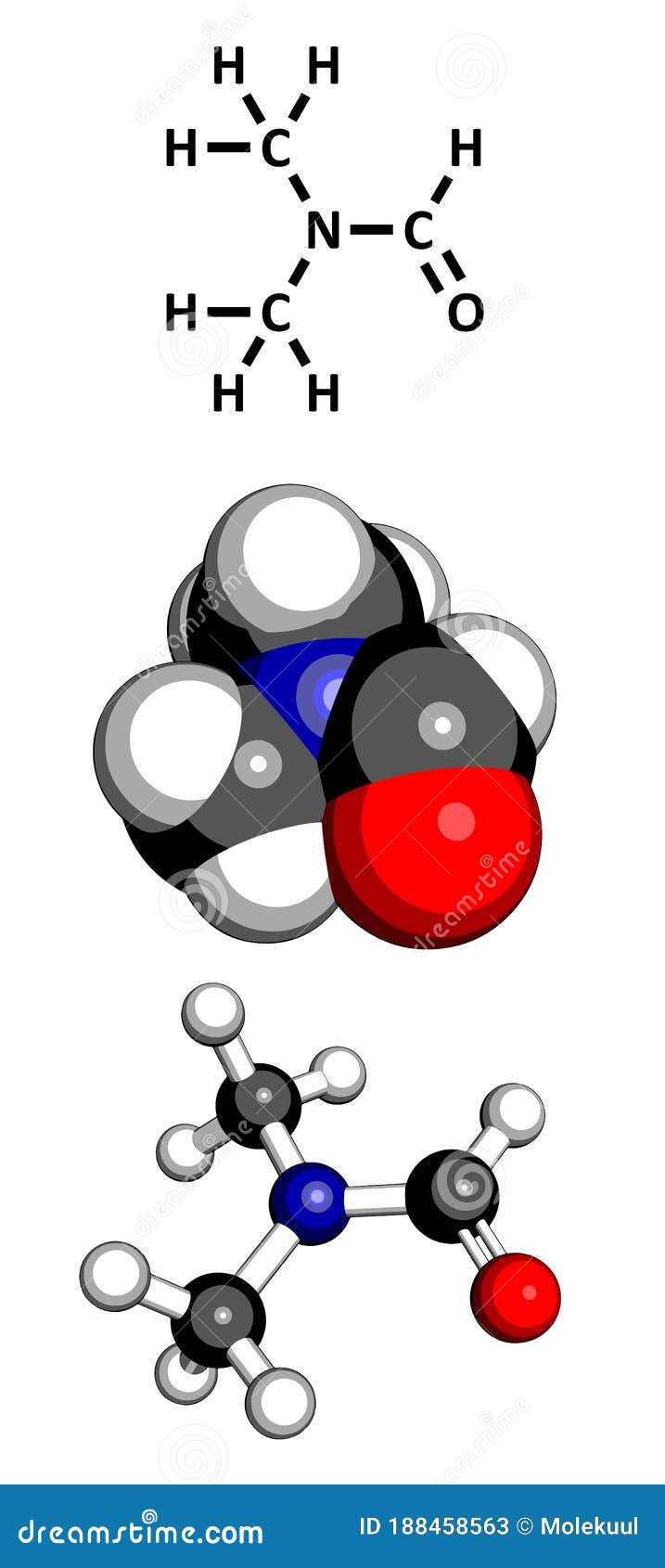
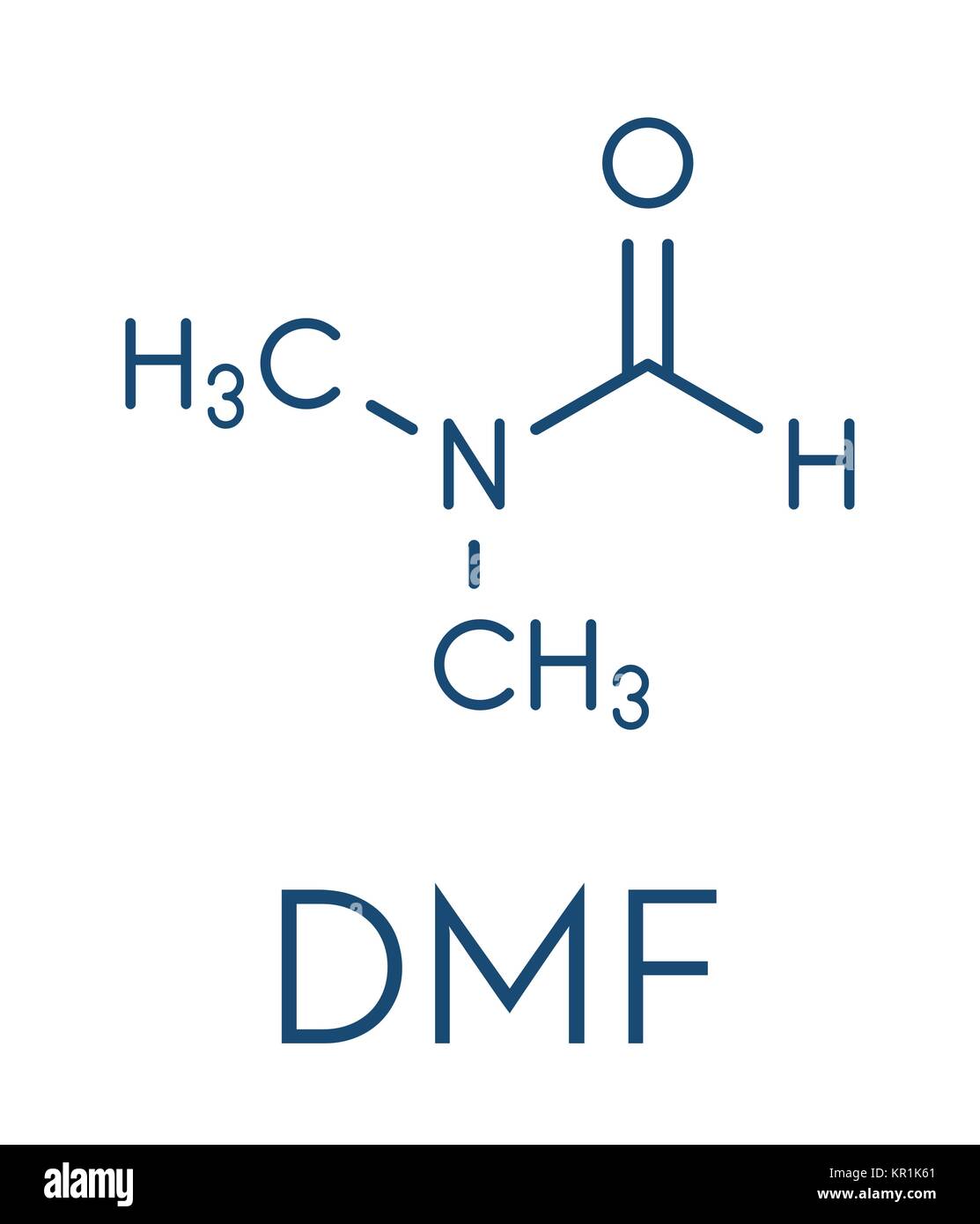
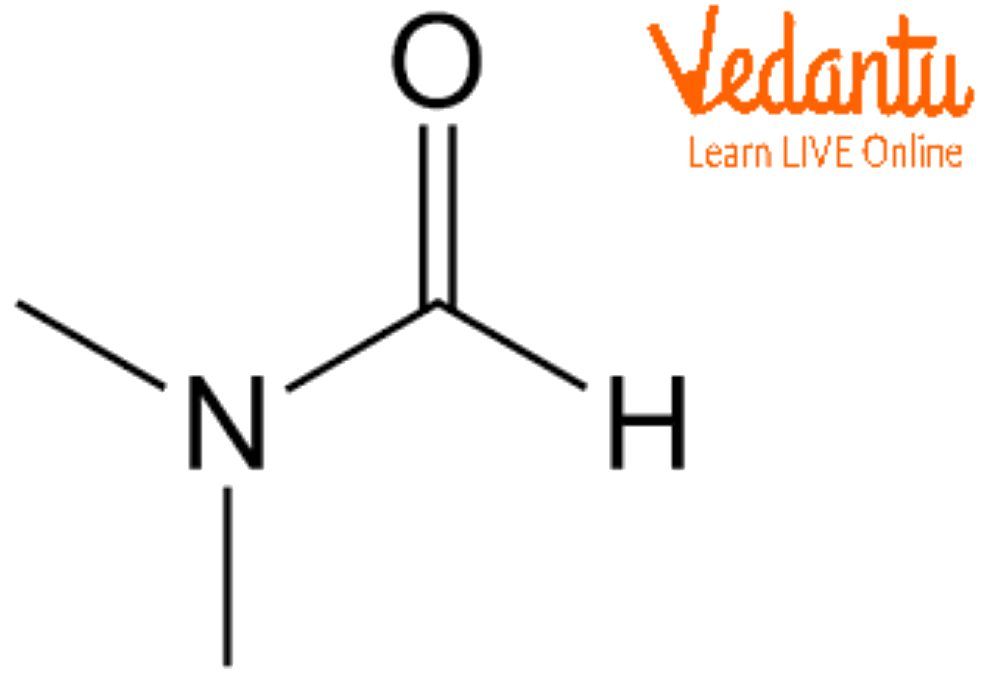
Calculating DMF’s Molecular Weight
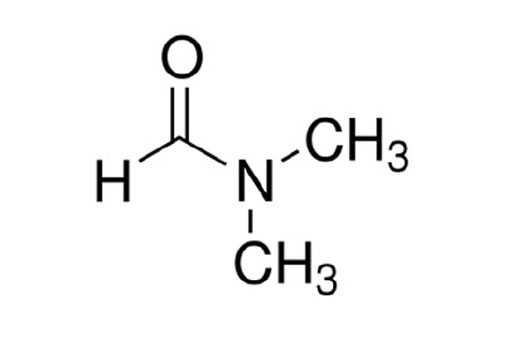
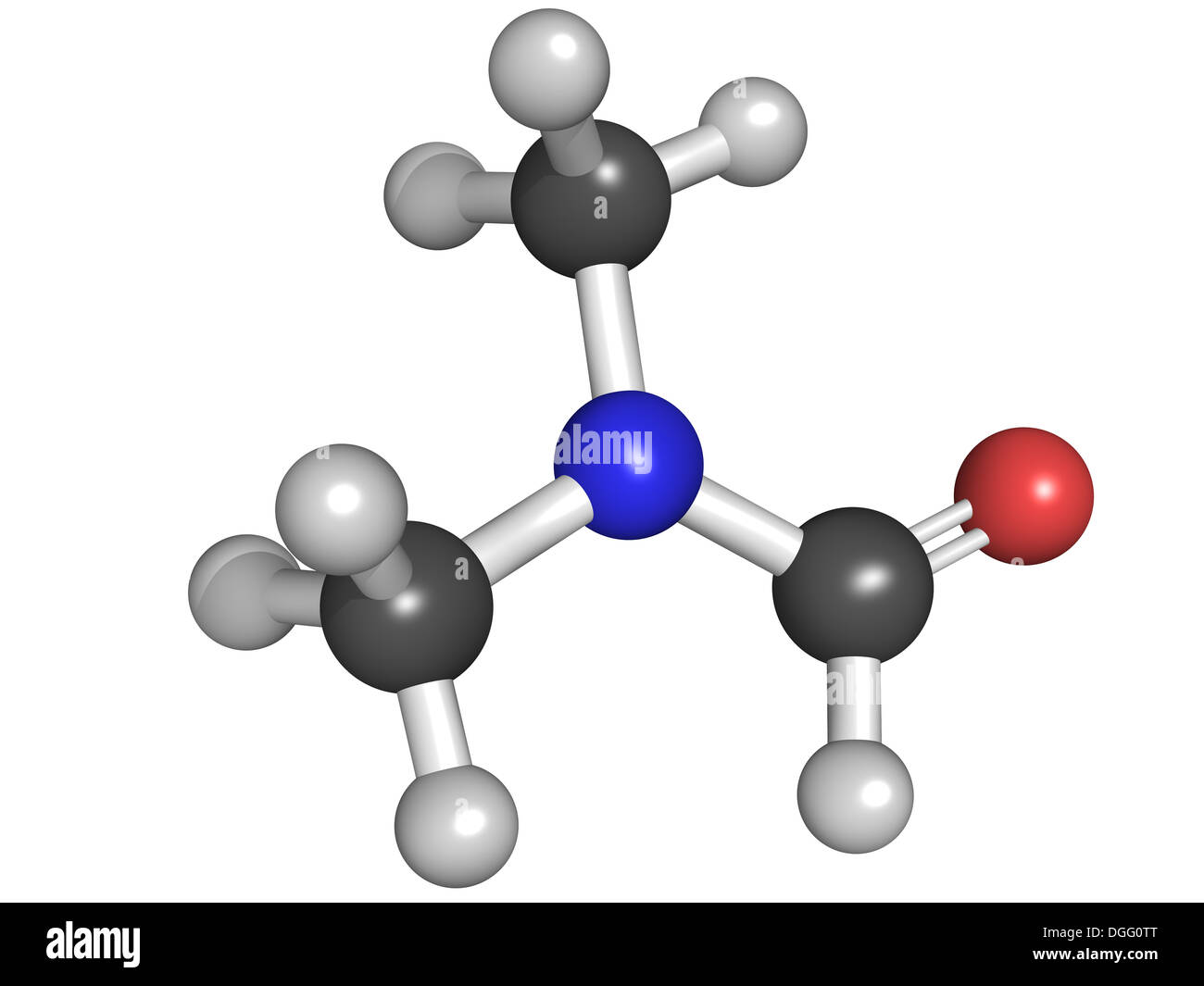
The chemical formula for DMF is (CH₃)₂NCHO. To calculate its molecular weight, we need to consider the atomic masses of each element present:
- Carbon (C): 12.01 amu
- Hydrogen (H): 1.01 amu
- Nitrogen (N): 14.01 amu
- Oxygen (O): 16.00 amu
By summing the atomic masses of all atoms in the DMF molecule, we arrive at:
- 2(12.01 amu) + 7(1.01 amu) + 14.01 amu + 16.00 amu = 73.09 amu
Therefore, the molecular weight of DMF is 73.09 amu or 73.09 g/mol.
The Importance of Molecular Weight in DMF Applications
DMF’s molecular weight is instrumental in various aspects of its applications, including:
- Solubility and Miscibility: DMF’s molecular weight influences its solubility in different solvents. Understanding this aspect is crucial for choosing suitable solvents for various reactions and processes.
- Reaction Stoichiometry: Precise knowledge of DMF’s molecular weight is essential for calculating the correct amounts of reactants and products in chemical reactions involving DMF as a solvent or reagent.
- Vapor Pressure and Volatility: DMF’s molecular weight affects its vapor pressure and volatility. This information is crucial for safety considerations, handling procedures, and process optimization.
- Density and Viscosity: DMF’s molecular weight influences its density and viscosity, which are important parameters for designing and optimizing equipment and processes.
- Spectroscopic Analysis: Molecular weight is crucial for interpreting spectroscopic data obtained from techniques like NMR and mass spectrometry, providing insights into the structure and composition of DMF-containing samples.
Benefits of Understanding DMF’s Molecular Weight
Understanding DMF’s molecular weight offers several benefits, including:
- Accurate Calculations: Precise calculations are essential for achieving desired outcomes in various applications. Knowing DMF’s molecular weight ensures accurate stoichiometric calculations and reliable experimental results.
- Improved Process Control: Understanding the relationship between DMF’s molecular weight and its properties allows for better process control, leading to optimized reaction conditions and increased efficiency.
- Enhanced Safety: Knowledge of DMF’s molecular weight contributes to safer handling procedures by facilitating accurate estimations of vapor pressure and volatility, crucial for minimizing exposure and potential hazards.
- Cost-Effectiveness: Accurate calculations and process optimization based on DMF’s molecular weight can lead to reduced material waste, minimizing costs and maximizing efficiency.
Applications of DMF and its Molecular Weight
DMF’s versatility as a solvent makes it indispensable in numerous applications, where its molecular weight plays a crucial role:
- Chemical Synthesis: DMF is a popular solvent in organic synthesis, facilitating various reactions like Grignard reactions, Wittig reactions, and Diels-Alder reactions. Its molecular weight is crucial for accurate stoichiometry and optimizing reaction conditions.
- Polymer Chemistry: DMF is widely used as a solvent in the production of polymers, including polyamides, polyurethanes, and acrylic polymers. Its molecular weight influences its ability to dissolve monomers and facilitate polymerization reactions.
- Pharmaceutical Industry: DMF finds extensive application in the pharmaceutical industry as a solvent for various drug formulations and intermediates. Its molecular weight is crucial for solubility considerations and accurate dosage calculations.
- Industrial Applications: DMF is employed in various industrial processes, including the production of pesticides, herbicides, and dyes. Understanding its molecular weight is vital for optimizing processes and ensuring product quality.
FAQs about DMF’s Molecular Weight
Q1: How does the molecular weight of DMF affect its solubility in water?
A1: DMF has a relatively high molecular weight and a polar structure. While it is miscible with water, its solubility is limited due to its relatively large molecular size.
Q2: What is the significance of DMF’s molecular weight in determining its vapor pressure?
A2: DMF’s molecular weight affects its vapor pressure. Higher molecular weight compounds generally have lower vapor pressures, meaning they are less volatile.
Q3: How does DMF’s molecular weight affect its viscosity?
A3: DMF’s molecular weight influences its viscosity. Generally, higher molecular weight compounds tend to have higher viscosities.
Q4: What is the relationship between DMF’s molecular weight and its density?
A4: DMF’s molecular weight is directly related to its density. Higher molecular weight compounds generally have higher densities.
Q5: How does the molecular weight of DMF affect its safety considerations?
A5: DMF’s molecular weight influences its volatility and potential for exposure. Understanding its molecular weight is crucial for implementing appropriate safety measures, including ventilation and personal protective equipment.
Tips for Working with DMF and its Molecular Weight
- Accurate Weighing: Use a precise balance to ensure accurate weighing of DMF, crucial for achieving desired stoichiometry in chemical reactions.
- Proper Storage: Store DMF in tightly sealed containers to minimize evaporation and ensure purity.
- Safe Handling: Always wear appropriate personal protective equipment, including gloves and eye protection, when handling DMF.
- Ventilation: Ensure adequate ventilation when working with DMF to minimize exposure to its vapors.
- Consult Safety Data Sheets: Always consult the safety data sheet (SDS) for detailed information on DMF’s properties, hazards, and safe handling procedures.
Conclusion
The molecular weight of DMF plays a crucial role in understanding its properties, optimizing its applications, and ensuring safe handling. By recognizing the significance of this fundamental parameter, scientists and engineers can leverage DMF’s versatility and optimize its use in diverse fields, from chemical synthesis and polymer chemistry to pharmaceuticals and industrial processes. Understanding and effectively applying the principles of molecular weight in the context of DMF contributes to efficient, safe, and sustainable practices across various industries.

Closure
Thus, we hope this article has provided valuable insights into Demystifying the Significance of Molecular Weight in Dimethylformamide (DMF). We hope you find this article informative and beneficial. See you in our next article!